Answer:
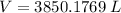
Step-by-step explanation:
Gas law is the mathematical relationship among pressure,volume, temperature, and the number of moles of a gas. It is the equation of state for an ideal gas. Which is because the state of a gas can be known by it pressure, volume, temperature, and number of moles.
Look below which "R" is a constant
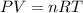
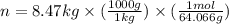
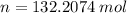
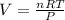
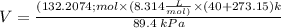
or (3.85 x 10^3 L)
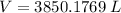
~Lenvy~