Answer:
D
Step-by-step explanation:
Given that the molar mass of NaCl is 58.44 g/mol, we want to determine the molarity of a solution that contains 87.75 g of NaCl in 500. mL of solution.
Recall that molarity is given by:
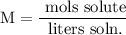
Therefore, we can convert the amount of NaCl in the solution to moles of NaCl and the volume from milliliters to liters:
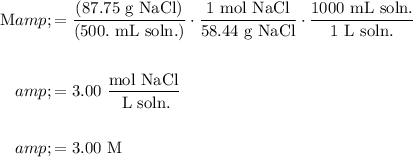
Hence, our answer is D.