- Atomic weight of copper = 63.5 g
- 1 mole of copper contains = 63.5g = 6.023 × 10²³ atoms of copper
- 1 g of copper contains
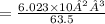 = 9.4 × 10²¹ atoms
= 9.4 × 10²¹ atoms- Therefore, 3.11g of copper contains
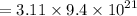
- = 29.234 × 10²¹ atoms
Hope you could get an idea from here.
Doubt clarification - use comment section.