- Mass of H₂O (water) is 89 g and we are asked to find number of molecules present in 89 g of H₂O.
Step-by-step explanation :-

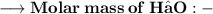

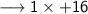

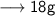
 ____________________
____________________
Let's calculate the number of moles present in 89g of H₂O.

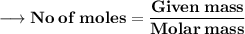

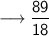

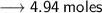
We know –
- Avogadro number = 6.022×10²³
Number of molecules:-

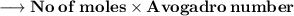

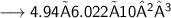

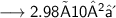

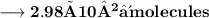
- Thus, 2.98×10²⁴ molecules are there in 89g of H₂O.
 ____________________
____________________