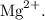Magnesium becomes an ion by losing two electrons from its outermost shell, which results in a
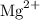 ion with a 2+ charge.
ion with a 2+ charge.
To answer the questions regarding how Magnesium becomes an ion and the charge of the Magnesium ion, we can follow these steps based on general chemistry knowledge:
1. Understand Magnesium's Electron Configuration: Magnesium (Mg) is an element with atomic number 12, meaning it has 12 protons and, when neutral, 12 electrons. Its electron configuration is
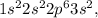 with the two 3s electrons being the outermost electrons.
with the two 3s electrons being the outermost electrons.
2. Ion Formation: Magnesium becomes an ion by losing electrons to achieve a more stable electronic configuration. The most stable electronic configuration is often that of a noble gas, which, for Magnesium, would be the configuration of Neon (Ne), with an electron configuration of
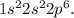
3. Losing Electrons: To achieve this configuration, Magnesium loses the two 3s electrons. This loss of electrons results in a positively charged ion because there are now more protons (12) than electrons (10).
4. Resulting Charge: After losing two electrons, Magnesium has a 2+ charge because it has two more protons than electrons. The resulting ion is denoted as