Final answer:
To find the mass of a single Mg atom we multiply its atomic mass (24 amu) by the mass of one atomic mass unit (
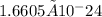 grams/amu), yielding approximately
grams/amu), yielding approximately
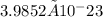 grams for one Mg atom.
grams for one Mg atom.
Step-by-step explanation:
To calculate the mass of one Mg atom, given its atomic mass is 24g, we will use the concept of atomic mass units (amu). One amu is defined as 1/12th the mass of a carbon-12 atom, which approximately equates to 1.6605 × 10-24 grams per amu. Therefore, the mass of one Mg atom, which has an atomic mass of 24 amu, can be calculated by multiplying the atomic mass by the mass of one amu.
Mass of one Mg atom = 24 amu × 1.6605 × 10-24 grams/amu
This calculation results in a mass of approximately 3.9852 × 10-23 grams for one magnesium (Mg) atom.