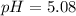
Explanation:
The pH of a solution can be calculated from its hydronium concentration using the equation below:
![pH = -\log [H_3O^+]](https://img.qammunity.org/2022/formulas/mathematics/college/3942tvalcegyigb5x37qaq0mdexpldxb4h.png)
where
![[H_3O^+]](https://img.qammunity.org/2022/formulas/mathematics/college/jp5o7ex9yefa5pn7ckdliddb9zw2a8i9sg.png) is the hydronium ion concentration in mol/liter unit. This notation is the same as the more familiar symbol
is the hydronium ion concentration in mol/liter unit. This notation is the same as the more familiar symbol
![[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/869xv4va2pom353pcqwbgfhb9dbdu5d2fd.png) for hydrogen ion concentration. Since the hydronium ion concentration is
for hydrogen ion concentration. Since the hydronium ion concentration is
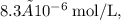 the pH value of the solution is
the pH value of the solution is
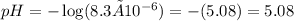
This means that the solution is acidic since its pH value is below 7.