Answer:
The enthalpy change of the reaction is 4.78 × 10⁴ J.
Step-by-step explanation:
We are given that 0.717 g of sodium metal reacts with hydrochloric acid to produce 7450 J of heat.
Converting grams of sodium to moles:
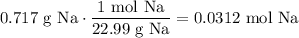
And dividing the amount of heat produced by the moles of sodium reacted yields:
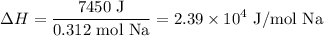
Because the given reaction has two moles of sodium metal, we can multiply the above value by two to acquire the enthalpy change of the given reaction:
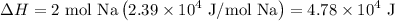
In conclusion, the enthalpy change of the reaction is 4.78 × 10⁴ J.