Answer:
Approximately
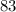 minutes.
minutes.
Step-by-step explanation:
Look up the relative atomic mass of
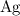 :
:
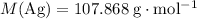 .
.
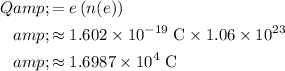 /.
/.
Avogadro's number:
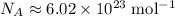 .
.
Elementary charge:
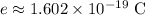 .
.
Calculate the quantity of
 atoms to reduce:
atoms to reduce:
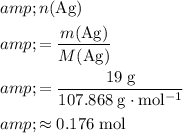 .
.
By the equation, it takes one electron to reduce every
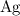 atom. Thus, the number of electrons required to reduce
atom. Thus, the number of electrons required to reduce
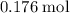 of
of
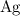 atoms would be:
atoms would be:
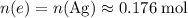 .
.
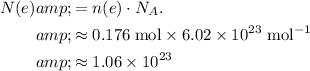 .
.
Calculate the amount of charge (in coulombs) in that many electrons:
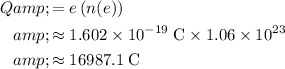 .
.
A current of
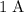 carries a charge of
carries a charge of
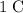 every second. Thus, the amount of time required for this current to carry that much electron would be:"
every second. Thus, the amount of time required for this current to carry that much electron would be:"
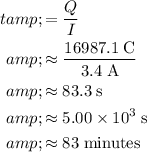 .
.