Answer:

Step-by-step explanation:
We are asked to find the volume of 0.25 moles of oxygen gas.
At standard temperature and pressure or STP, one mole of any gas has a volume of 22.4 liters.
We want to convert 0.25 moles of oxygen gas or O₂ to liters. We will use dimensional analysis, so we must set up a conversion factor.
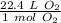
We are converting 0.25 moles of oxygen gas, so we multiply the conversion factor by this value.
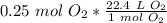
The units of moles of oxygen gas cancel.
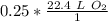
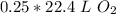
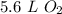
0.25 moles of oxygen gas occupy 5.6 liters at standard temperature and pressure.