Answer:

Step-by-step explanation:
We are asked to find how many atoms are in 9.35 moles of lithium (Li)
Moles are converted to atoms using Avogadro's Number or 6.022×10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are atoms of lithium.
We convert using dimensional analysis, so we set up a conversion factor using Avogadro's Number.
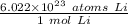
We are converting 9.35 moles of lithium to atoms, so we multiply the conversion factor by this value.
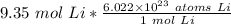
The units of moles of lithium cancel.
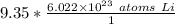
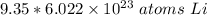
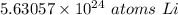
The original value of moles (9.35) has 3 significant figures, so our answer must have the same. For the number we found that is the hundredth place. The 0 in the thousandth place tells us to leave the 3 in the hundredth place.
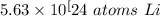
9.35 moles of lithium contains approximately 5.63×10²⁴ atoms of lithium.