Solution ⟹
Let u be velocity of hydrogen atom before collision and V the ve locity of two atoms moving together after collision By principal of conservation of momentum , we have
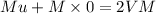
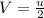
The loss in kinetic energy Δϵ due to collision is
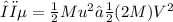
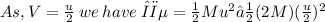
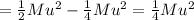
Rest answer is in the pic