Answer:
1.7 moles of CO
Step-by-step explanation:
First, you need to know that the volume occupied by 1 mole of gas at NTP is 22.4 L.
NTP means Normal Temperature and Pressure.
So if we have 38.5 liters of CO:
22.4 L --- 1 mol
38.5 L --- x mol
x =
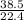
x = 1.7 moles of CO