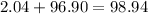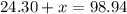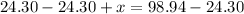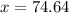Answer:
Hydrogen Chloride is 74.64 grams.
Step-by-step explanation:
Using the given information, we can find the amount of grams of hydrogen chloride that take place in the reaction.
- 24.30 grams of Magnesium
- "x" grams of Hydrogen chloride
- 2.04 grams hydrogen gas
- 96.90 grams of magnesium chloride.
Due to the Law of Conservation of Mass, the mass of the system must remain constant. To find the value of x, make the equation below.
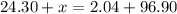
The left side represent the reactant side of the chemical reaction and the right side represents the product side.