The equation is given by;
2HCl => H² + Cl²
According to stoichiometry;
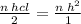
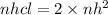
To produce 0.62 moles of H² gas we need,
nHCL=2×0.62=1.24 moles
Using the relation;
number of moles:Number of atoms/Avogadro's constant
We can then say;
N(numb. of atoms)=n×Na
Number of atoms=1.24×6.02×10²³
Number of atoms=7.5852×10²³ atoms