Answer:
Step-by-step explanation:
We are asked to find the density of an object.
The density of a substance is its mass per unit volume. It is calculated by dividing the mass by the volume.
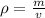
The mass of the object is 1.08 kilograms. The object displaced 50.50 cubic centimeters of water, so this value is its volume.
We are asked to give the density in grams per cubic centimeter, so we must convert the mass. There are 1000 grams in 1 kilogram. Set up a conversion factor.
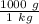
Multiply by the given mass: 1.08 kg
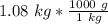
The units of kilograms cancel.
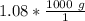
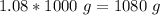
Now we know the mass in grams and the volume:
Substitute the values into the density formula.
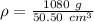
Divide.
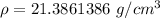
The original measurements of mass and density have 3 and 4 significant figures. Our answer must have the least number of significant figures, or 3.
For the number we found, that is the tenths place. The 8 in the hundredth place tells us to round the 3 in the tenths place up to a 4.
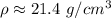
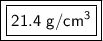
The density of the object is approximately 21.4 grams per cubic centimeter.