This is really simple. Assuming all these reactions are stoichiometric, the ratios of moles if always given by:
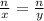
The first n represents the number of moles of the first reactant.
The x represents the coefficient of the first reactant in the equation.
The second n represents the number of moles of the second reactant.
The y represents the coefficient of the second reactant.
For example, let's use the first equation:
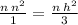
Using cross multiplication;
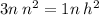
For each H² mole, we need 3 N² moles
Ratio of N² to H² is 3:1
Hope this given you an idea of how to continue the rest.
If you get stuck you can always comment so i can help you.
Make sure to tell me what you get as your answers so you get a good grade :)