Answer:

Step-by-step explanation:
1 mole of any gas (including CH₄ or methane) at standard temperature and pressure (STP) has a volume of 22.4 liters.
We will convert moles to liters using dimensional analysis, so we must set up a conversion factor using the information above.
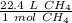
We are converting 2.35 moles of methane to liters, so we multiply the ratio by 2.35 moles.
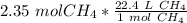
The units of moles of methane cancel.
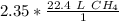
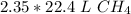
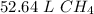
The original value of moles (2.35) has 3 significant figures, so our answer must have the same.
For the number we found, that is the tenths place. The 4 in the hundredth place tells us to leave the 6 in the tenths place.
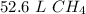
2.35 moles of methane have a volume of 52.6 liters.