Answer:
C
Step-by-step explanation:
The reaction between carbon dioxide and lithium hydroxide can be represented by the equation:
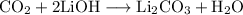
We want to determine the amount of moles of LiOH needed to react completely with 25.5 g of CO₂.
Determine the number of moles of CO₂ in 25.5 g of CO₂:
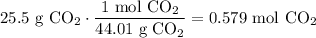
Use stoichiometry to determine the amount of LiOH necessary. CO₂ and LiOH are in a 1:2 ratio. That is, for every one mole of CO₂ that reacts, two moles of LiOH must also react.
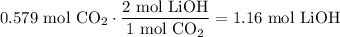
In conclusion, our answer is C.