Answer:
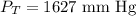
Step-by-step explanation:
Dalton's law of partial pressures states that the total pressure in a mixture of gases is the sum of the partial pressures of the individual gases.
In other word:
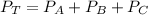
Therefore, the total pressure of the mixture of three gases is:
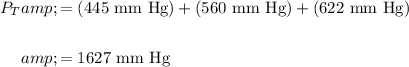
In conclusion, the total pressure of the mixture is 1627 mm Hg.