Answer:
237.98
Step-by-step explanation:
U-238 (99.2745%), U-235 (0.7200%), U-234 (0.0055%).
Multiply mass with percentage of abundance
238 × 99.2745 = 23627.331 %
235 × 0.72 = 169.2 %
234 × 0.0055 = 1.287 %
Add all the percentages.
23627.331 + 169.2 + 1.287 = 237978.818 %
As a whole number it is =
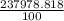
Average atomic mass of Uranium= 237.98 [rounded to 2 decimal places]