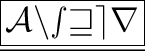
Positive and negative ions attract each other and combine with each other to yield ionic compounds and they are separated easily to conduct electricity.
- The concentration of ions in pure water is very less and is almost unionised, thus does not conduct electricity.