Answer:
Initial Volume ( V₁ 0.43L
Initial Concentration (M₁) 13M
Final Concentration ( M₂) 3.0M
Final Volume ( V₂) = ?
we have to determine the final volume of diluted solution .
Using Dilution Equation
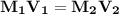
Put the given value

Therefore,
- 1.86 L of the diluted solution will produced .