Answer:
Mass of substance m = 0.050Kg
Initial Temperature T₁ = 20°C
Final Temperature T₂ = 100°C
we have to calculate the heat absórbed by the lead .
Since spècific heat of lead is not gíven so we will take spècific heat of lead 128 J/kg°C
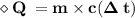 Put the given value
Put the given value

Therefore,
- Heat absórbed by the lead is 512 Joules.