Answer:
The molar mass of the precipitate is 233.39 g/mol.
Step-by-step explanation:
We are given that a solution of sodium sulfate (Na₂SO₄(aq)) is mixed with a solution of barium nitrate (Ba(NO₃)₂(aq)). And we want to determine the molar mass of the precipitate this is formed.
Write the balanced double-replacement reaction. Note that all sodium and nitrate ions are soluble and BaSO₄ is insoluble. Hence:
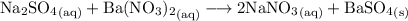
From the equation, we can see that the precipitate that is formed is barium sulfate.
The molar mass of barium sulfate is:
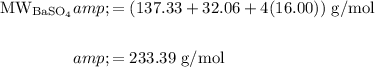
In conclusion, the molar mass of the precipitate is 233.39 g/mol.