Answer:
Question 1 :
• A compound is ionic if it is made up of a metal or a cation (+) and a non metal or anion (-)
Question 2 :
• While naming ionic compounds, follow the formula → "metal" + "non-metal ending with ide "
• i.e; Sodium Chloride:
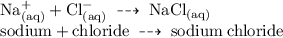
Question 3 :
• The answer above that question is perfect.
Question 4 :
1 atom → Mono. But it is highly recommended to ignore it
2 atoms → DI
3 atoms → TRI
4 atoms → TETRA
5 atoms → PENTA ( such as pentaoxide )
7 atoms → HEPTA ( such as heptaoxide )
Question 5 and 6:
Are perfectly answered.