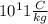Answer:
A) the same in all gases.
Step-by-step explanation:
The Thomson's experiment showed that the charge-to-mass ratio of electron was Same in all gases.
J.J. Thomson in his Cathode ray experiment showed that the atoms are made of aggregate charged particles. Until his demonstration it was believed that atoms were smallest unit. In his calculation he also showed that charged to mass ratio is same in all the elements.
 ×
×