Answer:
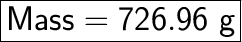
Step-by-step explanation:
In the reaction:
For 2 moles of Na, 1 mol of H₂ is produced
At STP,
1 mol of H₂ = 22.4 L/mol molar value
Converting 354 L to moles:
= volume / molar value
= 354 / 22.4
= 15.8 moles
We already know that:
1 mol of H₂ ↔ 2 moles of Na
So,
15.8 moles of H₂ ↔ 15.8 × 2 moles of Na
15.8 moles of H₂ ↔ 31.6 moles of Na
So, to produce 15.8 moles of H₂ (354 L), we need 31.6 moles of Na.
Converting 31.6 moles of Na into mass:
Mass = moles × molar mass
Mass = 31.6 × 23
Mass = 726.96 g
![\rule[225]{225}{2}](https://img.qammunity.org/qa-images/2023/formulas/biology/high-school/vdifidejf5i8c49g0hg7.png)