Answer:
The density of the sample is 11.8 g/mL.
Step-by-step explanation:
We are given an unknown sample with a mass of 23.5 g and a volume of 2.00 mL and we want to determine its density.
Recall that density is given by:
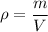
Where ρ is the density, m is the mass, and V is the volume.
Substitute 23.5 g for m and 2.00 mL for V and evaluate:
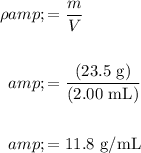
Hence, the density of the sample is 11.8 g/mL.