Magnesium combined with chlorine.
How?
- Look at the question .Compound has -1 charge in it .Hence it contains Halogen.
- Looking at the options Option B and C contain Iodine and chlorine i.e halogens .
Now come to metals side.
- Potassium has valency 1(Look the Electronic configuration below)
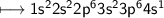
Hence it will contain +1 charge which is omitted directly .
Now come to option C.
- Magnesium has charge +2(Look at EC below
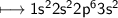
Hence the compound formed is given by
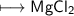
Hence option C is correct.