Answer:
41.0 L CO₂
Step-by-step explanation:
We want to determine the volume of carbon dioxide gas present in a 80.55 gram sample at STP.
To do so, we can convert from grams to moles and then use the STP ratio to determine its volume.
Find the molar mass of CO₂:
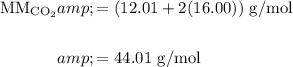
And recall that for any gas at STP, there are 22.4 L/mol.
Dimensional Analysis:
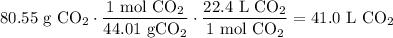
In conclusion, there will be about 41.0 L of carbon dioxide gas at STP in a 80.55 gram sample.