Answer:
8 grams
Step-by-step explanation:
- Finding the mr of each part:
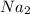 = 23 x 2 = 46
= 23 x 2 = 46
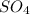 = 32.1 + (16 x 4) = 96.1
= 32.1 + (16 x 4) = 96.1
- Finding the fraction of the compound that is Na+
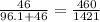
- We now know that per gram, 460/1421g are Na+
In 25g,
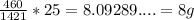
So there are 8g Na+
Hope this helps, let me know if you have any questions! :)