Answer:

Step-by-step explanation:
We are asked to find how many molecules are contained in 4.00 grams of carbon dioxide.
1. Grams to Moles
Convert grams to moles using the molar mass (mass of 1 mole of a substance). The molar mass of carbon dioxide is given: 44.00 grams per mole.
We will convert using dimensional analysis. Set up a conversion factor using the molar mass.
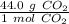
We are converting 4.00 grams of carbon dioxide to moles, so we multiply by this value.
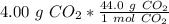
Flip the conversion factor. The value remains the same, but it allows us to cancel the units of grams of carbon dioxide.
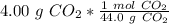
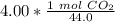
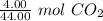
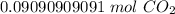
2. Moles to Molecules
Convert moles to molecules using Avogadro's Number or 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are molecules of carbon dioxide.
Set up another conversion factor, this time with Avogadro's Number.
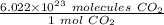
We are converting 0.09090909091 moles of carbon dioxide to molecules, so we multiply by this value.
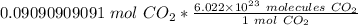
The units of moles of carbon dioxide cancel.
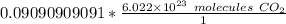
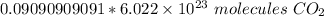
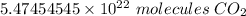
3. Significant Figures
The measurement of molar mass (44.00) has 4 sig fig and the measurement of molecules (4.00) has 3 sig fig. We round to the least number of sig fig, or 3.
For the number we found, that is the hundredth place. The 4 in the thousandth place tells us to leave the 7.
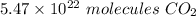
4.00 grams of carbon dioxide contains 5.47 × 10²² molecules of carbon dioxide.