Answer:
A = 0.75 gram or 1 gram
Explanation:
The half-life of carbon 14 is years. How much would be left of an original -gram sample after 2,292 years? (To the nearest whole number).
We can use the following formula for half-life of
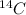 to find out how much is left from the original sample after 2,292 years:
to find out how much is left from the original sample after 2,292 years:
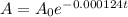
where:
A is the amount left of an original gram sample after t years, and
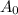 is the amount present at time t = 0.
is the amount present at time t = 0.
The half-life of
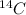 is the time t at which the amount present is one-half the amount at time t = 0.
is the time t at which the amount present is one-half the amount at time t = 0.
If 1 gram of
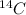 is present in a sample,
is present in a sample,
Solve for A when t = 2,292:
Substituting
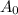 = 1 gram into the decay equation, and we have:
= 1 gram into the decay equation, and we have:
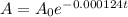
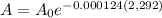
A = 0.75 g or 1 g