ANSWER:
4 a) Specific elements have more than one oxidation state, demonstrating variable valency.
For example, the following transition metals demonstrate varied valence states:
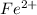 ,
,
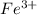 ,
,
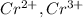 , etc.
, etc.
Normal metals such as
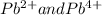 also show variable valencies. Certain non-metals are also found to show more than one valence state
also show variable valencies. Certain non-metals are also found to show more than one valence state
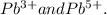
4 b) Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
For example, Carbon-14 is a naturally occurring radioactive isotope of carbon, having six protons and eight neutrons in the nucleus. However, C-14 does not last forever and there will come a time when it loses its extra neutrons and becomes Carbon-12.
5 a)
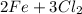 →
→
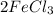
5 b)
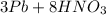 →
→
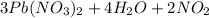
5 c)
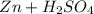 →
→
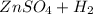 (already balanced so don't need to change)
(already balanced so don't need to change)
5 d)
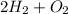 →
→
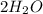
5 e)
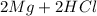 →
→
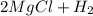
EXPLANATION (IF NEEDED):
1. Write out how many atoms of each element is on the left (reactant side) and right (product side) of the arrow.
2. Start multiplying each side accordingly to try to get atoms of the elements on both sides equal.
EXAMPLE OF BALANCING: