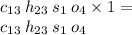Answer:
C atoms= 13
H atoms= 23
S atoms= 1
O atoms= 4
Step-by-step explanation:
No. of C moles=
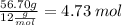
No. of H moles=
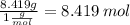
No.of S moles=
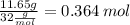
No.of O moles=
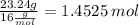
So the ratios are,
C : H : S : O
4.73 : 8.419 : 0.364 : 1.4525
13 : 23 : 1 : 4
So the empirical formula will be
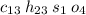
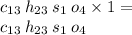
To find the final answer
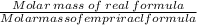
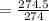
It approximately equals 1
So,