Let's find the masses of carbon and hydrogen from the masses of our products
for carbon:
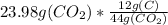
mass of carbon = 6.54 gram
for hydrogen:
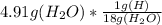
mass of hydrogen = 0.27 gram
Total mass of carbon and hydrogen:
Mass of Carbon + Mass of Hydrogen = 6.54 + 0.27 = 6.81 gram
since we had a 10 gram sample, the rest of the mass must be because of Oxygen. so,
Mass of Oxygen:
Mass of compound - Mass of carbon and hydrogen
10 - 6.81 = 3.19 gram Oxygen
Finding number of moles:
Moles of Carbon:
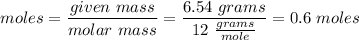
Moles of Oxygen:
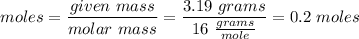
Moles of Hydrogen:
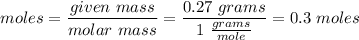
Empirical Formula:
Carbon : Hydrogen : Oxygen
0.6 : 0.3 : 0.2
6 : 3 : 2
C₆H₃O₂