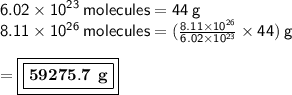Answer:
It is 59,275.74 grams
Explanation:
• Molecular mass of carbon dioxide
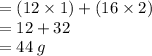
[ molar masses: C = 12 g, O = 16 g ]
• From avogadro's number, it says 1 mole contains 6.02 × 10^23 molecules or atoms.
But 1 mole is equivalent to its molar mass in terms of weight, therefore;