Answer:
3.54 L
Step-by-step explanation:
To find the new volume, we'll use this formula:
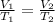
V₁ = Liters (5.6)
T₁ = Volume in kelvin (273 + 35 = 308)
V₂ = New liters (?)
T₂ = New temperature (273 - 78 = 195)
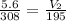
First, cross multiply
1092 = V₂308
Divide both sides by 308
1092/308 = V₂308/308
V₂ = 3.54