Answer:
393 mL
Step-by-step explanation:
Recall the ideal gas law:
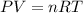
Because only pressure and volume are changing and everything else remains constant, we have that:
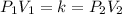
Where k is some constant.
Hence, substitute and solve:
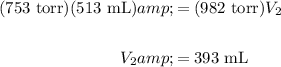
Hence, the new volume is 393 mL.
Check: because volume decreases with an increase in pressure, our answer fits within the context of the problem.