Answer:
B. False
Step-by-step explanation:
In an exothermic reaction, the average enthalpy change is a negative value ( loss in energy ).
since enthalpy change of reaction is given by:
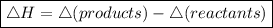
❎ when enthalpy energy of products is higher than that of reactants, ∆H will be positive ( endothermic reaction ).
✔ When enthalpy energy of products is lower than that of reactants, ∆H will be negative ( exothermic reaction ).