Answer:

Step-by-step explanation:
We are asked to find how much heat is required to raise the temperature of a sample of water. We are given the mass and temperature, so we will use the following formula.
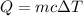
The mass (m) of the sample is 96 grams. The specific heat capacity (c) of water is 4.18 Joules per gram degree Celsius. The difference in temperature (ΔT) is found by subtracting the initial temperature from the final temperature.
- ΔT= final temperature - initial temperature
The temperature was raised from 35 °C to 68 °C.
Now we know three variables and can substitute them into the formula.
- m= 96 g
- c= 4.18 J/g °C
- ΔT= 33°C
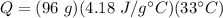
Multiply the first two numbers together. The units of grams cancel.
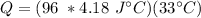
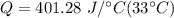
Multiply again. The units of degrees Celsius cancel.
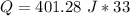
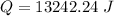
The original measurements of mass and temperature have 2 significant figures, so our answer must have the same. For the number we calculated, that is the thousands place. The 2 in the hundreds place tells us to leave the 3.
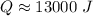
Approximately 13,000 Joules of heat are required.