Answer:
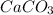
Step-by-step explanation:
This is because calcium is a metal that forms ions with a 2+ charge (as it has 2 electrons on its outer shell).
Carbonate ions are complex ions with a 2- charge, so with one carbonate and one calcium, the 2+ and 2- charges will cancel out.