Answer:
Approximately
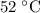 (approximately
(approximately
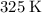 ), assuming that nitrogen is an ideal gas.
), assuming that nitrogen is an ideal gas.
Step-by-step explanation:
- Let
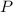 denote the pressure of this nitrogen gas sample.
denote the pressure of this nitrogen gas sample. - Let
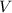 denote the volume of this nitrogen gas sample.
denote the volume of this nitrogen gas sample. - Let
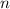 denote the number of moles of
denote the number of moles of
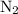 molecules in this nitrogen gas sample.
molecules in this nitrogen gas sample. - Let
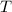 denote the absolute temperature of this nitrogen gas sample (typically measured in degrees kelvins.)
denote the absolute temperature of this nitrogen gas sample (typically measured in degrees kelvins.)
Let
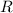 denote the ideal gas constant. By the ideal gas law, the following equation would relate these quantities:
denote the ideal gas constant. By the ideal gas law, the following equation would relate these quantities:
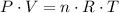 .
.
Rearrange this equation to obtain an expression for
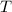 :
:
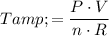 .
.
Look up the ideal gas constant:
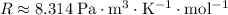 .
.
Convert each measurements from the question to standard units:
Substitute these values into the expression for
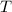 :
:
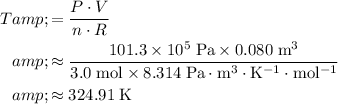 .
.
Convert the unit of this temperature to degrees celsius:
 .
.