Now lets she s-subshell structure .
Take an element Berylium
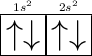
You can see the S-orbital has only space for 2electrons .
- So K shell has 2electrons
Come to p-orbital
Take an element Neon
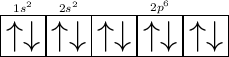
The 2 means its L shell .
It has 8 electrons only and full filled .
- Hence L shell can have only 8electrons