Answer:
22:
Formular:
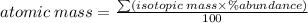
substitute:
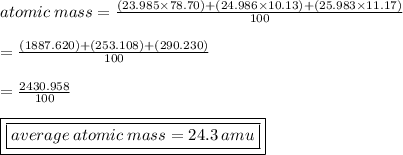
23:
Same element is represented by same number of protons.
Answer:
6 protons. 6 protons
7 neutrons. 8 neutrons
6 electrons. 6 electrons
Note: Atoms with same proton number but different mass number are called isotopes