The entire question says:
"A rock with a mass off 44.18 grams is dropped into a gratued cylinder. The water level in the cylinder rises from 15.20cm³ to 21.72cm³."
a) Calculate the volume of the rock.
The volume of the rock is equal the difference between the water level with the rock and the water level without the rock. That happened because when you put the rock inside the cylinder, the volume of it has been increased, and the volume of the rock is the value that increased. So, let's do this account:
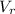 is the volume of the rock.
is the volume of the rock.
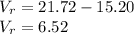
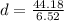
Therefore, the volume of the rock is 6.52cm³
b) Calculate the density of the rock.
To calculate the density of some material, we should divide the mass of the material by its volume. Below, you can see the formula:
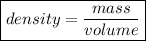
Now that we know about it, it is easier to solve our question. We just do this count. The volume is 6.52cm³ and the mass is 44.18g.
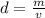
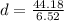
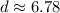
So, the density of this rock is approximately 6.78g/cm³.
I hope I've helped. ^^
Enjoy your studies! \o/