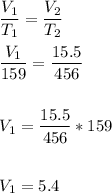Answer:
5.4 m³
Step-by-step explanation:
Charles Law: The volume of an ideal gas is directly proportional to the temperature when the pressure is kept constant.
V₁ -> initial volume ; T₁ - > initial temperature
V₂ - > final volume ; V₂ - > final temperature.