When we are adding water (or solvent) to a solution to decrease its concentration, we are diluting the solution. When dealing with dilution we will use the following equation:
M1 V1 = M2V2
M1 = initial concentration
V1 = initial volume
M2 = final concentration
V2 = final volume
Calculate the volume of the 1.75 M K2CrO4 stock solution needed
M1 = 0.100 M
V1 = 2.00 L
M2 = 1.75 M
V2 = ?
Remember the equation that I said
M1 V1 = M2 V2
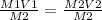
V2 =
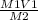
V2 = 0.100*2/1.75
0.114 L
We would prepare 2.00 L of 0.100 M K2CrO4 from 1.75 M K2CrO4 stock solution by diluting 0.114 L of stock solution to the desired volume of 2.00 L
Good Luck!