Answer:
» An electron is lighter than a proton.
Step-by-step explanation:
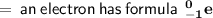
hence it's mass number is zero

hence it's mass number is 4
Therefore, proton is heavier than electron
» An electron has a small charge magnitude than a proton.
Step-by-step explanation:
An electron has charge of -1 while proton has charge of +2, therefore electron is less deflected by any energetic fields than a proton